The international Blood transfusion Genomics Consortium launches program to expand cutting-edge genomics for more accurate blood typing, leading to improved transfusion therapy and better outcomes.
NEW YORK (June 15, 2021) — Blood centers from around the world have united to form an international collaboration with academic research institutions and a leading genomic array company, Thermo Fisher Scientific, to deliver a technology which promises to make the future of blood transfusion therapy more efficient, cost-effective, accurate, standardized, and safer.
These organisations are collaborating as members of the recently formed Blood Transfusion Genomics Consortium, which brings together opinion leaders in the treatment of anaemia and other blood cell disorders, blood transfusion, computer science, population statistics and genomics. The main aim of the Consortium in this age of genomic medicine, is to design and validate for clinical use an affordable, single DNA-based blood typing test to improve the match between blood donors and the patients who receive blood transfusions. By working collaboratively between 10 countries across 4 continents, the adaptation of this new, genomics-based approach will, for the first time, set international standards for testing and use of blood transfusion therapy with the ultimate goal of improving the safety and efficacy of blood transfusion for the millions of patients we serve (Figure 1). The consortium participants hope to open up broader national and international access to a new generation of blood type analyses using genomic technologies to improve patient care around the globe.
Each year, millions of donations of blood provide life-saving treatment to patients all over the world. Transfusion is essential to save the lives of patients with major bleeding (e.g. trauma), to treat patients whose blood production is impaired or reduced by chemotherapy (e.g. cancer), and to manage patients with inherited anemia who do not make sufficient hemoglobin A (e.g. sickle cell disease).
Red blood cells have molecules on their surfaces called antigens, which are inherited and differ between individuals. Current practice is to match blood for transfusion according to the patient’s major ABO and Rh blood groups, for which donor blood is currently tested. Antigens of the many other blood groups are not routinely matched unless the patient has a reaction, because the current tests are too expensive. Hence, mismatches occur between the transfused donated cells and the cells of those from the patient. For some, this can cause life-threatening reactions in which the transfused cells are rapidly destroyed by the immune system (antibodies) of the patient.
Dr. Connie Westhoff from the New York Blood Center commented: “After decades of research, we are now on the cusp of introducing a cutting-edge genomic technology into routine practice. This is an exciting development and will make it possible to find compatible blood for the many patients requiring regular transfusions, particularly those with sickle cell disease.”
Blood types are determined by approximately 40 different genes. Consequently, DNA taken from a blood sample can be tested to determine the complete (more than ABO and Rh) blood type profile. The same test also allows us to rapidly type other tissue (HLA) and platelet (HPA) groups, which can help identify the best matched platelets to give to patients.
Dr. Gleadall from Cambridge University Hospitals and NHS Blood and Transplant said: “It is amazing to see how our long-standing collaboration with Thermo Fisher Scientific has resulted in the development of a new method for testing the genes for blood groups, HLA and HPA types which is cost-effective and can be applied to both patient and donor blood samples.”
Using samples from 8,000 blood donors, the results from the newly designed test have been compared with those of tests currently used by blood centers, with excellent agreement (99.9% concordance). Of note, almost half of the small number (73) of differences were caused by manual record entry errors in the results for the currently used tests (Figure 2).
Professor Ellen van der Schoot from the Sanquin Blood Supply Foundation in the Netherlands said: “What is really exciting is that this new test results in a 10-fold increase in the number of available antigen types and is easy to implement allowing for its wide use. With data from patients with complex antibodies it has been shown that this increased typing information makes it more likely that a donation for safe transfusion can be identified. Indeed, when the results of the new test obtained with the samples of 3,000 Dutch donors became available, Sanquin was able to provide life-saving transfusions to a young female patient for whom compatible blood could not be identified.”
To bring this precision medicine test to the bedside, operational validation and regulatory approval will be required. Consortium researchers have developed a pre-clinical study protocol with precise timelines in anticipation of regulatory approvals by international health authorities. By using the samples and antigen typing data of almost 100,000 blood donors from the 7 blood centers collaborating in this study, it is anticipated that industry acceptance and regulatory approval can be completed over a 24-month period. Once validated, the test will be used to support clinical trials at hospitals supported by the seven blood centers participating in the Consortium, in anticipation of commercial introduction of the test platform internationally.
Figure 1: The 10 member organisations of the Blood Transfusion Genomics Consortium
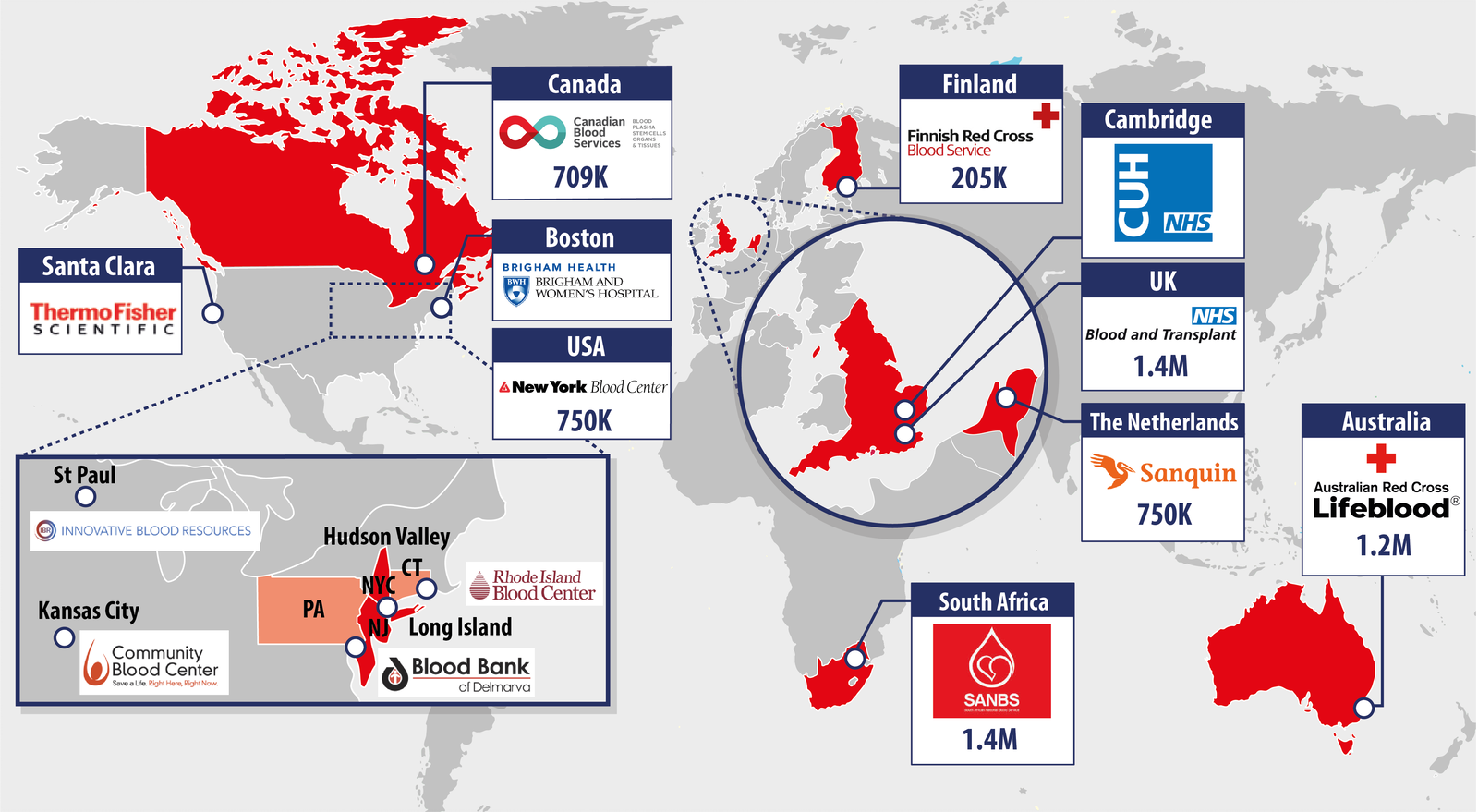
The national blood services of Australia, Canada, England, Finland, the Netherlands and South Africa together with the New York Blood Center work in partnership with academic hospitals in Boston and Cambridge, and Thermo Fisher Scientific to introduce cutting-edge genomic technology into routine clinical practice. Jointly the seven blood services collect 6.4 million donations of blood from donors per year to support the transfusion needs for a population of 162 million citizens.
Figure 2: Results of Pre-Clinical Study I performed by the Blood Transfusion Genomics Consortium
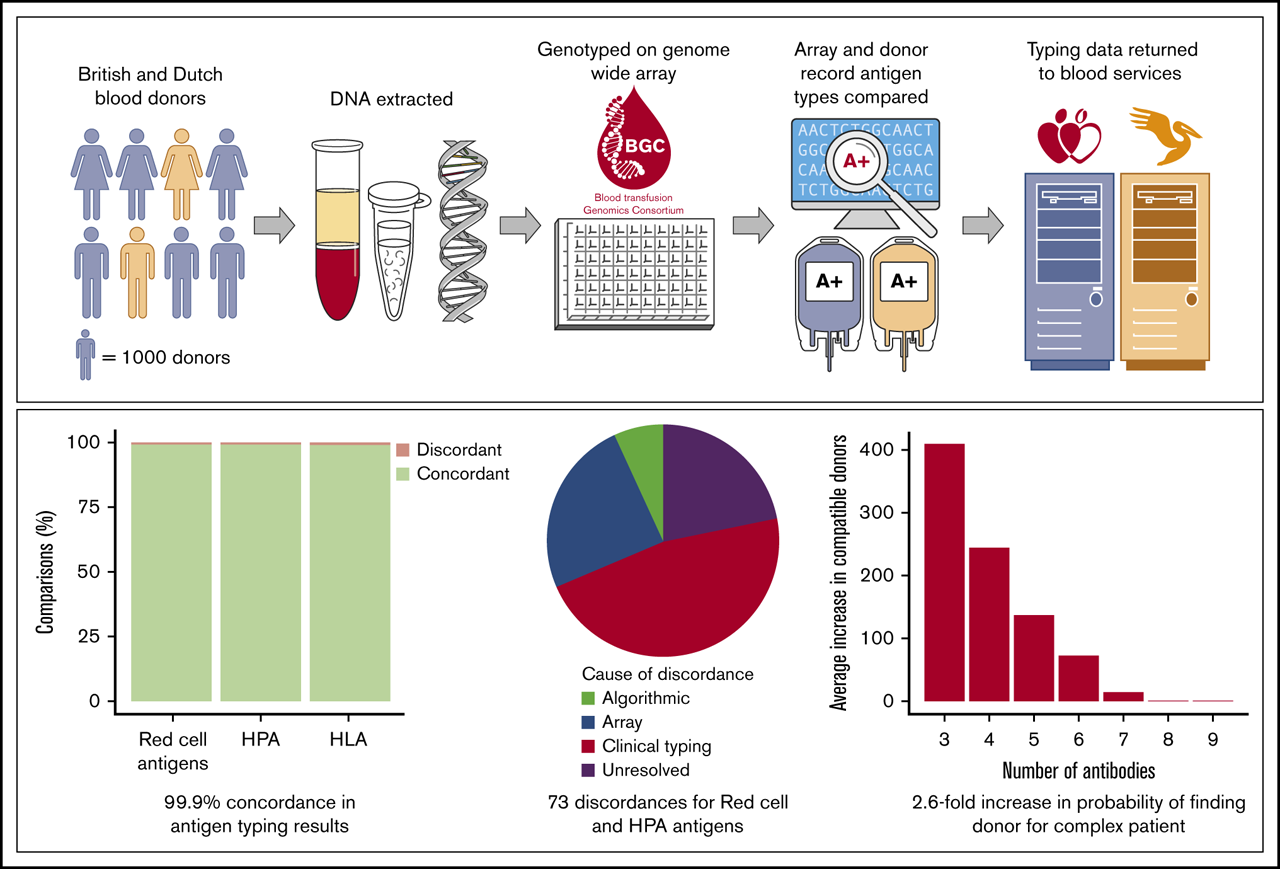
This study showed the power of the genomics array test to determine the blood groups, HLA and HPA types of donors with excellent accuracy (Gleadall et al., Blood Advances 2020. DOI: 10.1182/bloodadvances.2020001894)